The cyclic utilization of carbon resources can be achieved by the electroreduction reaction of CO2((CO2RR) into higher-value C2+(c≥2)诸如C之类的产品2H4,这是一种环保技术。
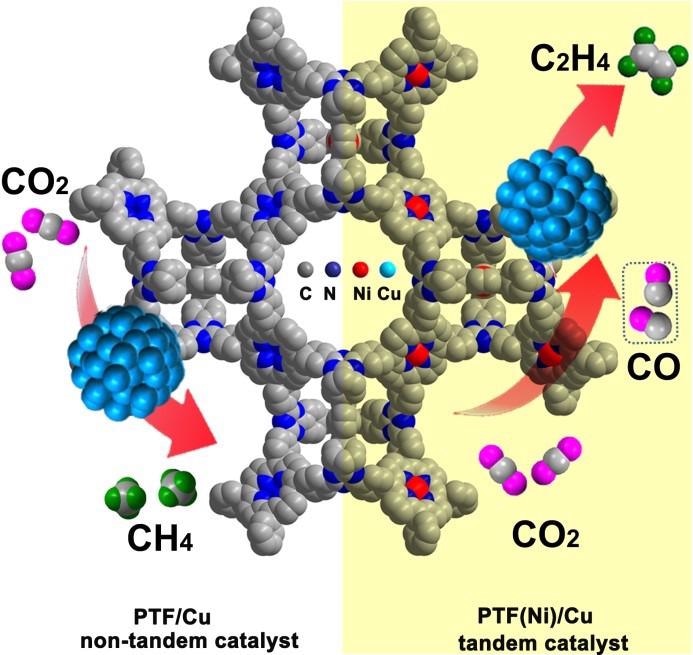 The tandem electroreduction of CO2to ethylene over atomically isolated nickel-nitrogen site/copper nanoparticle catalysts. Image Credit: Prof. Cao’s group.
The tandem electroreduction of CO2to ethylene over atomically isolated nickel-nitrogen site/copper nanoparticle catalysts. Image Credit: Prof. Cao’s group.
但是,高价值C的选择性和活性2+(c≥2)产品受到多电子传输过程和非常缓慢的C-C耦合步骤的高度限制。
福吉安(FujianChinese Academy of Sciences(CAS)现在已经设计了一种有效的串联催化方法来增强CO的选择性2RR toward C2H4by various unique catalytic sites in the local vicinity. The study was reported inAngewandte Chemie国际版。
The team developed an earth-abundant element–based tandem electrocatalyst PTF(Ni)/Cu. This was achieved by uniform dispersing of Cu nanoparticles (NPs) on the porphyrinic triazine framework that was anchored with atomically separated nickel-nitrogen sites (PTF(Ni)).
At -1.1 V, the Faradaic efficiency of C2H4与可逆的氢电极(RHE)相比,触摸57.3%,该氢电极(RHE)比非tandem Catalyst PTF/CU(卟啉三嗪框架固定在没有金属的情况下)的近六倍,超过了大部分催化剂。
PTF(Ni)/Cu表现出最佳稳定性,并连续合成C2H4在电解11小时,如几乎不变的总电流密度和FEC所证明的2H4。From the electroreduction of CO (CORR) on PTF/Cu and PTF(Ni)/Cu, it was proven that the in-situ generated CO through atomically separated nickel-nitrogen sites is highly significant. This can shift quickly to the closer Cu NPs for the subsequent C-C coupling reactions to synthesize C2H4。
此外,Operando ATR-FTIR和密度功能理论(DFT)计算表明,CU位点上较高的局部CO浓度导致 *CO CO中间覆盖率增加了Cu表面。这改善了C-C耦合活力,从而改善了C的形成2H4。By contrast, a low CO concentration favored the synthesis of CH4。
这项研究提出了一种简单而常见的合成方法,以基于单原子活性位点开发串联催化剂。这种方法可以进一步指导新一代高效CO的设计2用于生产多碳产品的RR催化剂。
期刊参考:
Meng, D.-L.,et al。((2021) Highly Selective Tandem Electroreduction of CO2在原子分离的镍 - 硝基部位/铜纳米颗粒催化剂上进行乙烯。Angewandte Chemie国际版。doi.org/10.1002/anie.202111136。
Source:https://english.cas.cn/